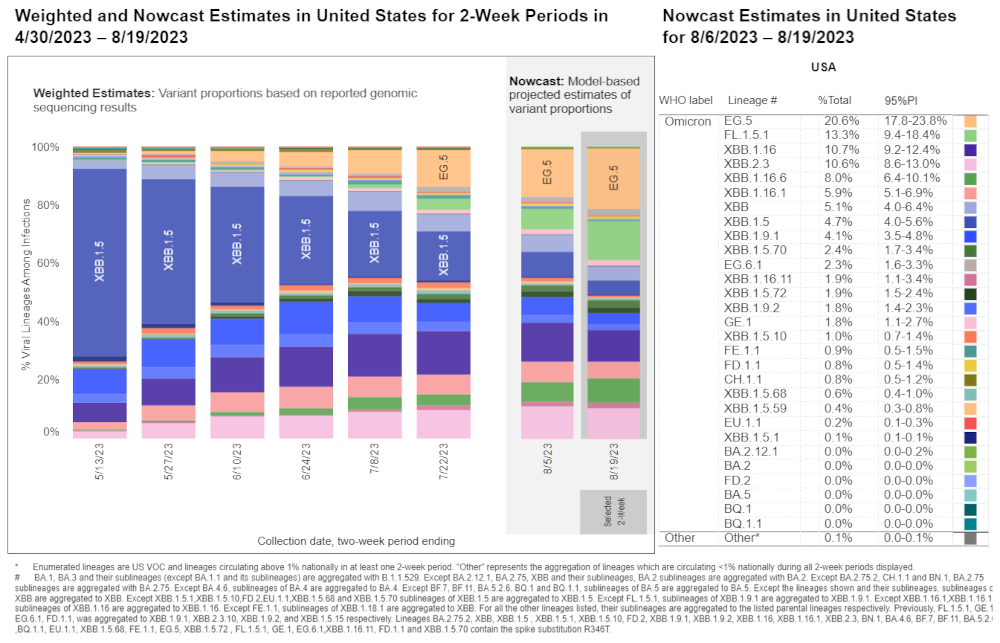
The XBB.1.5 Strain Declines as EG.5 Grows in Prevalence
Part of the reason we need a new booster against COVID-19 every year is that SARS-CoV-2 is a fast-evolving virus. As you can see from the Centers for Disease Control data below, in May the XBB.1.5 variant made up the majority of COVID-19 cases, but by mid-August, XBB.1.5 had become a small portion of cases, with the new EG.5 strain, first detected in February, on its way to dominance. Additionally, a new strain, designated BA.2.86, has gained a foothold in the U.S. and is being closely watched by doctors and epidemiologists.
You can read more about EG.5 at the New York Times, the Washington Post, ABC News, CNN or Yale Medicine. You can read more about the BA.2.86 strain at CBS News or Yale Medicine. The CDC’s variant tracker is here.
Advisory Committee on Immunization Practices Will Meet September 12
The Advisory Committee on Immunization Practices (ACIP), a group of independent experts that advises the Centers for Disease Control and Prevention, will be meeting on September 12 to consider approval of a new round of vaccines against COVID-19. The ACIP is expected to approve three COVID-19 vaccines: two mRNA vaccines, from Pfizer and Moderna, and a third protein subunit vaccine from Novavax. All three will be monovalent vaccines (vaccinating for a single virus) against the XBB.1.5 strain. Though XBB.1.5 is being rapidly displaced by the EG.5 strain (see previous story), it is expected that the vaccine will be effective against closely related strains.
You can read about the ACIP meeting and the new vaccine at CBS News and CNN. Details of the meeting have been posted in the Federal Register and the ACIP meeting will be webcast live from 10:00 AM – 4:00 PM Eastern on the 12th via the ACIP website here.
COVID-19, RSV, flu Trio Recommended for Fall
This Fall will be a watershed in the struggle against respiratory infection, with experts recommending a trio of vaccines against COVID-19, RSV and the flu. Particularly notable is the RSV vaccine, as this is the first time a vaccine has ever been approved for RSV. The vaccine, Arexvy, made by GSK, was approved in June. In a study of 25,000 people, the vaccine was 94% effective at preventing severe disease in seniors. Though RSV is most often associated with children, 160,000 people 65 and older are hospitalized each year with RSV, and 10,000 to 13,000 die from the infection. Three additional vaccines against RSV – by Pfizer, Moderna and Bavarian Nordicare – are also nearing approval.
You can read about the trio of vaccines in articles in NBC News and CNN. The development of a vaccine for RSV has been a scientific quest 60 years in the making. You can learn some of the history of the effort to create an RSV vaccine in the CNN article about its approval.